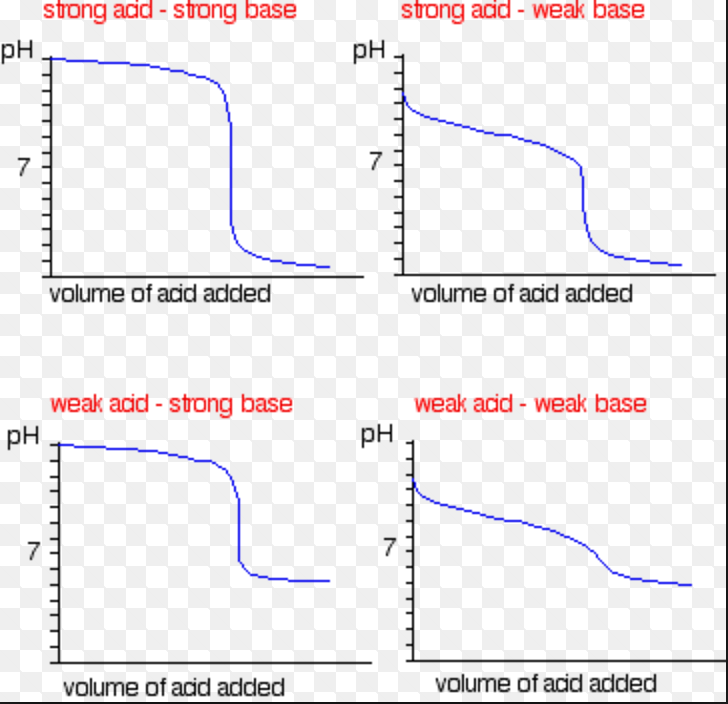
Titration Examples Chemistry . Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown. The example below demonstrates the. Titration is a widely used technique to measure the amount of one. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Before we start discussing about. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The concentration of an acid solution can be determined by titration with a strong.
from classnotes.org.in
The concentration of an acid solution can be determined by titration with a strong. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Before we start discussing about. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown. Titration is a widely used technique to measure the amount of one. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The example below demonstrates the.
Acid Base Titration using Indicator Chemistry, Class 11, Ionic
Titration Examples Chemistry The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Before we start discussing about. The concentration of an acid solution can be determined by titration with a strong. Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a widely used technique to measure the amount of one. The example below demonstrates the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base.
From mavink.com
Titration Labeled Titration Examples Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a widely used technique to measure the amount of one. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration is the slow addition of one solution. Titration Examples Chemistry.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Examples Chemistry Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown. The example below demonstrates the. The above equation works only for. Titration Examples Chemistry.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Examples Chemistry The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration is a widely used technique to measure the amount of one. Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown. A titration is. Titration Examples Chemistry.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube Titration Examples Chemistry Before we start discussing about. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The concentration of an acid solution can be determined by titration. Titration Examples Chemistry.
From mungfali.com
Acid Base Titration Calculation Titration Examples Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration refers to a process where the use of a solution of known concentration takes place for the determination of. Titration Examples Chemistry.
From quizizz.com
Titration Method Chemistry Quiz Quizizz Titration Examples Chemistry A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The example below demonstrates the. Titration refers to a process where the use of a solution. Titration Examples Chemistry.
From mssmithsechs.weebly.com
Acids, Bases, Solutions, and Titrations MS. SMITH'S CLASS Titration Examples Chemistry The concentration of an acid solution can be determined by titration with a strong. Titration is a widely used technique to measure the amount of one. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration is the slow addition of one solution of a known concentration (called a. Titration Examples Chemistry.
From www.youtube.com
Back Titration Example YouTube Titration Examples Chemistry The example below demonstrates the. Before we start discussing about. Titration is a widely used technique to measure the amount of one. Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown. The concentration of an acid solution can be determined by titration with a. Titration Examples Chemistry.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Examples Chemistry The concentration of an acid solution can be determined by titration with a strong. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Before we start discussing about. Titration refers to a process where the use of a solution of known concentration takes place for the determination of the. Titration Examples Chemistry.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Examples Chemistry Before we start discussing about. The concentration of an acid solution can be determined by titration with a strong. Titration is a widely used technique to measure the amount of one. The example below demonstrates the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration refers to a. Titration Examples Chemistry.
From saylordotorg.github.io
AcidBase Titrations Titration Examples Chemistry Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The example below demonstrates the. Before we start discussing about. The. Titration Examples Chemistry.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Examples Chemistry Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown. The concentration of an acid solution can be determined by titration with a strong. The example below demonstrates the. Before we start discussing about. A titration is a laboratory technique used to precisely measure molar. Titration Examples Chemistry.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Examples Chemistry Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a widely used technique to measure the amount of one. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The concentration of an. Titration Examples Chemistry.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Examples Chemistry The concentration of an acid solution can be determined by titration with a strong. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration refers. Titration Examples Chemistry.
From www.youtube.com
Titration calculation example Chemistry Khan Academy YouTube Titration Examples Chemistry Titration is a widely used technique to measure the amount of one. The concentration of an acid solution can be determined by titration with a strong. Before we start discussing about. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Titration is the slow addition of one solution of. Titration Examples Chemistry.
From mungfali.com
Acid Base Titration Formula Titration Examples Chemistry Titration is a widely used technique to measure the amount of one. The example below demonstrates the. Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown. The concentration of an acid solution can be determined by titration with a strong. Before we start discussing. Titration Examples Chemistry.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration Examples Chemistry The concentration of an acid solution can be determined by titration with a strong. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The example below demonstrates the. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid. Titration Examples Chemistry.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Titration Examples Chemistry Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The example below demonstrates the. Titration is a widely used technique to measure the amount of one. The above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the. Titration Examples Chemistry.